(주)에스켐텍
Colloidal Silica
01 Particle & Surface
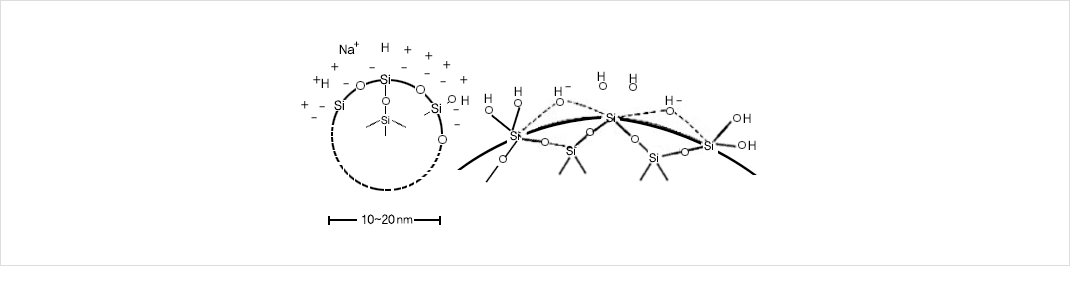
- Silica surface is charged by hydroxyl ions formed by loss of protons from water molecules in the spaces between the oxygen atoms of the structures.
- According to this electric charge of particles, it has formed gelation, aggregation and colloid. If the particles are smaller than 7nm in diameter the sol is almost as clear as water.
- From 10nm to 30nm there is a characteristic opalescence or transparency when seen it. And above about 50nm the appearance is white and milky.
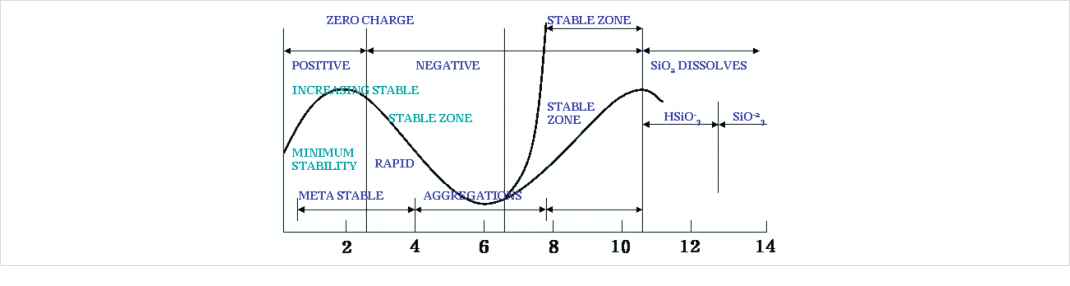
02 Effect of pH
03Use of SS-SOL
-
Increasing the coefficient of friction
Increasing the friction of solid surfaces. For example, railway tracks, waxed floors, and textile fibers. -
Adhesion, Film
Forming strong and rigid solid surface. -
Stiffening
Because it can be produced rigid gel by sintering, it is used for stiffening powder. -
Increasing cohesion by Gelation
It can increase cohesion, formed uniform gel by gelation. And it can be dispersed. Therefore it can be stabilized supporter -
Increasing hydrophilicity & prevention electrification
Hydrophilicity can be increased by Si-OH, and electrification can be prevented. -
Increasing activity
Because of lower ion concentration and higher silica concentration, when liquid is mixed reaction is uniform. And activity rate is faster. -
Lager surface
After dried colloidal silica, it can be got silica gel large that has surface area and uniform pore. -
Impregnant & Filler
It is used for filler of porous substance. And it is environmental harmony because of lower Na+ concentration.
04 Making Catalysts, Gels, Asdorbents

The advantage of a catalyst base of colloidal silica spheres in a densely packed arrangement is that at elevated temperature, the mass cannot readily contract further and collapse or sinter, and the uniform pores between the uniformly packed uniform particles provide a constant surface area and high degree of catalytic activity.
The silica sol containing metal salts can be spay-dried or freeze-dried to give small spherical gel particles which can be further compacted as desired. Sol can be converted to a fine powder by dispersing the sol in a partly water-miscible organic solvent, gelation the silica, and distilling off the liquids.
05 Inorganic Binder, Stiffener

-
Molded Refractory Bodies
A mullite bonded refractory was made by mixing colloidal silica and basic aluminum chloride in proportions to form mullite and using this as a binder for mullite powder to form a mullite refractory body at 1300℃. Refractory bodies of silllimanite have been bonded with a mixture of colloidal silica and basic aluminum chloride acting as a binder for sillimanite powder, fired at 1300~1400℃. Cold-molded metal bodies with thermal conductivity can be made from metal powders using colloidal silica and latex as the binder. -
Binders for Fibers
An inorganic binder for inorganic fibers is made by dispersing clay in colloidal silica, then acidifying to pH 3.5 and adding an aluminum salt such as aluminum formate. Fire-resistant reflective insulating material is made by binding fibrous potassium tianate with a mixture of latex and colloidal silica. Colloidal silica is used as binder in highly refractory aluminosilicate fibers. The stiffness and strength of organic fiber sheets or papers can also be improved with colloidal silica. In paperboard used for corrugating, stiffness is improved by impregnating with colloidal silica. From 1 to 5% colloidal silica in certain paper pulps gives improvement in strength, stiffness, stability, etc.
06 Frictionizing Effets

-
Fibers
Colloidal silica is coated with a cationic quaternary ammonium type of surfactant before application to textiles for good frictionizing. Because of its fractionizing effects, silica is also an aid in processing wool. Slippage of glass fibers is prevented and colors are simultaneously bonded to the surface by the application of colored metal oxides along with colloidal silica and heating to bond the coating. To prevent knots in nylon fishnets from slipping, colloidal silica is mixed with and water applied to the knots. -
Paper & Film
The surface of polyethylene terephthalate drafting film is improve with respect to reception of pencil
and ink by applying colloidal silica along with an acid-soluble film-forming material.
07 Antisoiling Surface
08 Hydrophilizing Surfaces
Transfer of oil-printing is prevented by a spray of colloidal silica. A hydrophilic nature of “planographic” paper printing plates is preserved or renewed by colloidal silica. A hydrophilic film of colloidal silica contains dispersed ink-receptive material, which is released to the surface by pressure or heat to form printing areas. A similar effect is involved in planographic offset masters.
09 Modifying Adhesion

-
Increasing Adhesion
If silica particles are anchored to or embedded in a surface, the submicroscopic roughness and polarity of the surface are increased and adhesion of a second material is generally improved: on the other hand, if the silica is present as a loose. Friable coating, or is applied as mixture with a silicone or fluorocarbon polymer, adhesion is reduced. Silica particles are embedded into the surface of polyethylene film to improve the adhesion of coatings of thermoplastic polymers. A fluorocarbon polymer surface is made cementable by coating it with the mixture of dispersed polytetrafluoroethylene and colloidal silica, and heating the surface to over 500℃ for a few minutes. A film of polyethylene is strongly adherent if heated against paper coated with colloidal silica. A coating composition of polytetrafluoroethylene contains alkali metal silicate and colloidal silica to improve adhesion and electrical insulating properties on metal surfaces. First treating the fiber with colloidal silica and heating to just below the fusion temperature improve the adhesion of colored coatings on glass fibers.
10 Coating Compoitions
11 Reinforcing Organic Polymers

12 Polishing Agent for Silicon Wafers

13 Miscellaneous Optical Effects, Color, Photography
14Use in Biological Research-Density Gradient
15 Sounrce of Chemistry Reactive silica
-
Soluble Silicates
Silica rapidly depolymerizes in the presence of strong alkali. Thus colloidal silica can be converted to a solution of sodium polysilicate containing from 4.2 to 6.0 moles of silica per mole of sodium oxide or lithium polysilicate and lithium silicate is not soluble in hot water. For lower ratio silicates the advantage of starting with colloidal silica is only a matter of convenience because of the rapid reaction rate. -
Glass Compositions
Homogeneous glasses can be made by admixing the other components intimately with colloidal silica. The chemical reactivity of colloidal silica plays a role in colored or conductive coatings on glass or refractory materials in which a vitrified bond is developed without damaging the substrate. -
Forming Solid Silicates-Cements
The oldest known reaction of amorphous silica is that in Raman cement where lime was mixed with sand and colloidally subdivided silica of volcanic origin mined at Pozzuoli, Italy, and Greek island of Santorini. This was the basis of the extremely impervious cement linings used in cisterns throughout the Mediterranean area and in construction throughout the Raman Empire without which some of the vast domes could never have been built. Sand and lime alone do not form such cement.
주소 : 경기도 안산시 단원구 해봉로 101
Tel : 031-492-4951 | Fax : 031-492-4954 | E-mail : sale@shsilicate.com
Copyright ⓒ (주)에스켐텍 All rights reserved.


